JCA update available
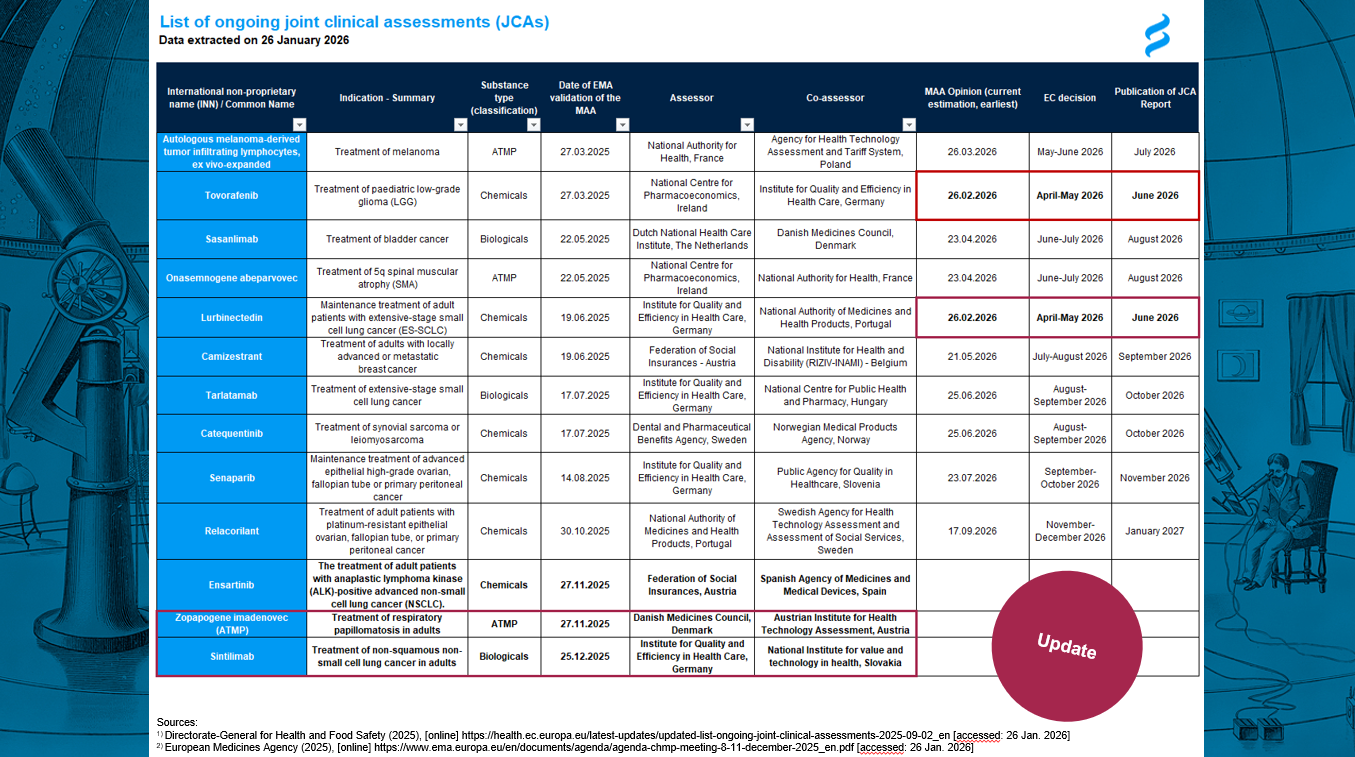
📢 JCA update available
💡 We provide you with the latest regulatory update from the EMA, announced last week.
🟥 Find the updates in the red frames 🟥
In summary:
🔹Tovorafenib: no submission of responses to day 180 immediately, expected submission in these very days – after 1 month of clock-stop. This leads to a JCA report published in June 2026.
🔹 Lurbinectedin: submission of responses to day 120 on 28 November 2025 (after only 1 month clock stop) and now listed in the CHMP January meeting agenda for adoption of day 180 “List of Outstanding Issues”. In case of immediate submission of responses immediately, the JCA report may be published in June 2026 as well.
🔹 Senaparid: the applicant requested an extension of clock-stop, therefore the procedure outcome may be delayed.
🔹 There are three new MAA/JCA procedures started in November/December 2025.
🔭 Take a look at the analysis in our observatory.
🙏 Thanks to our partners for the regulatory insights.
Disclaimer: Our analysis is compiled using only publicly available information and assumes a standard EMA timeline of 3 months clock stop d120 and 1 month d180, except where clock stop extensions are agreed with EMA. Subject to change without notice.
We do not guarantee the accuracy of the information provided here.