Tool News PART 10: The European HTA landscape in one database
🔧 PART 10: The European HTA landscape in one database
Series: monthly news from the EU HTA workshop
🔍 Oncology products and Advanced Therapy Medicinal Products (ATMPs) are among the first drugs subjected to EU HTA. This brings new opportunities as well as risks for successful market access across Europe plus country-level challenges for reimbursement.
🧭 However, navigating the European HTA landscape and collecting data across European countries is not easy and requires a lot of time!
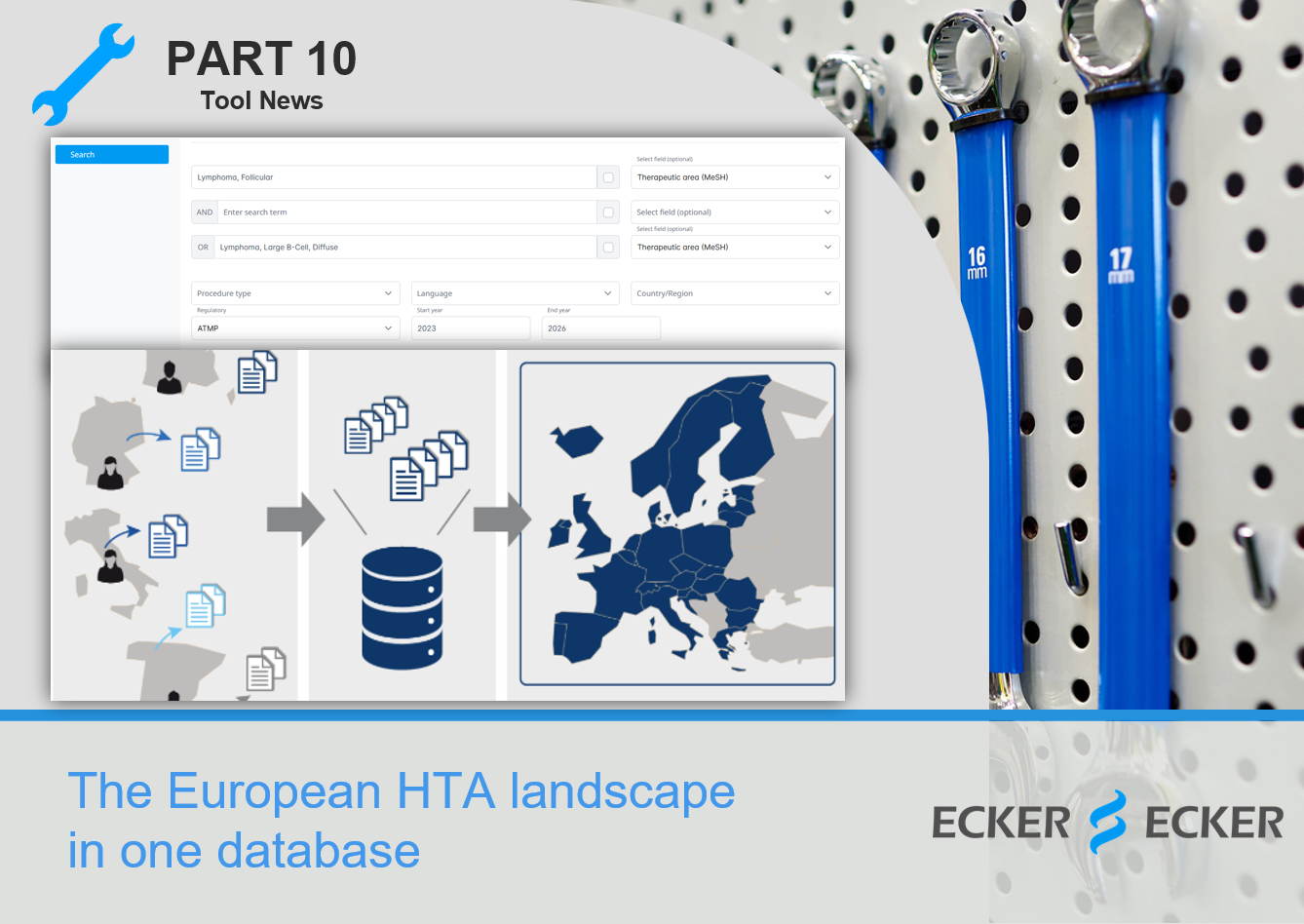
Looking for a shortcut❓️
The European HTA database by our brand Accessus Health offers fast access to the information you need, bundled in one place:
🔹 country-specific data,
🔹 curated by local experts,
🔹 following one common methodology,
🔹 a comprehensive and consistent overview of relevant HTA documents,
➡️ to support preparation for EU HTA assessments and all European and local market access activities.
👀 Want to take a closer look?
👉 Test our EU HTA database yourself: https://www.accessus-health.eu/european-hta-database